Publications
Full list of publications avaliable at Google Scholar
Research Articles
Science | 2020
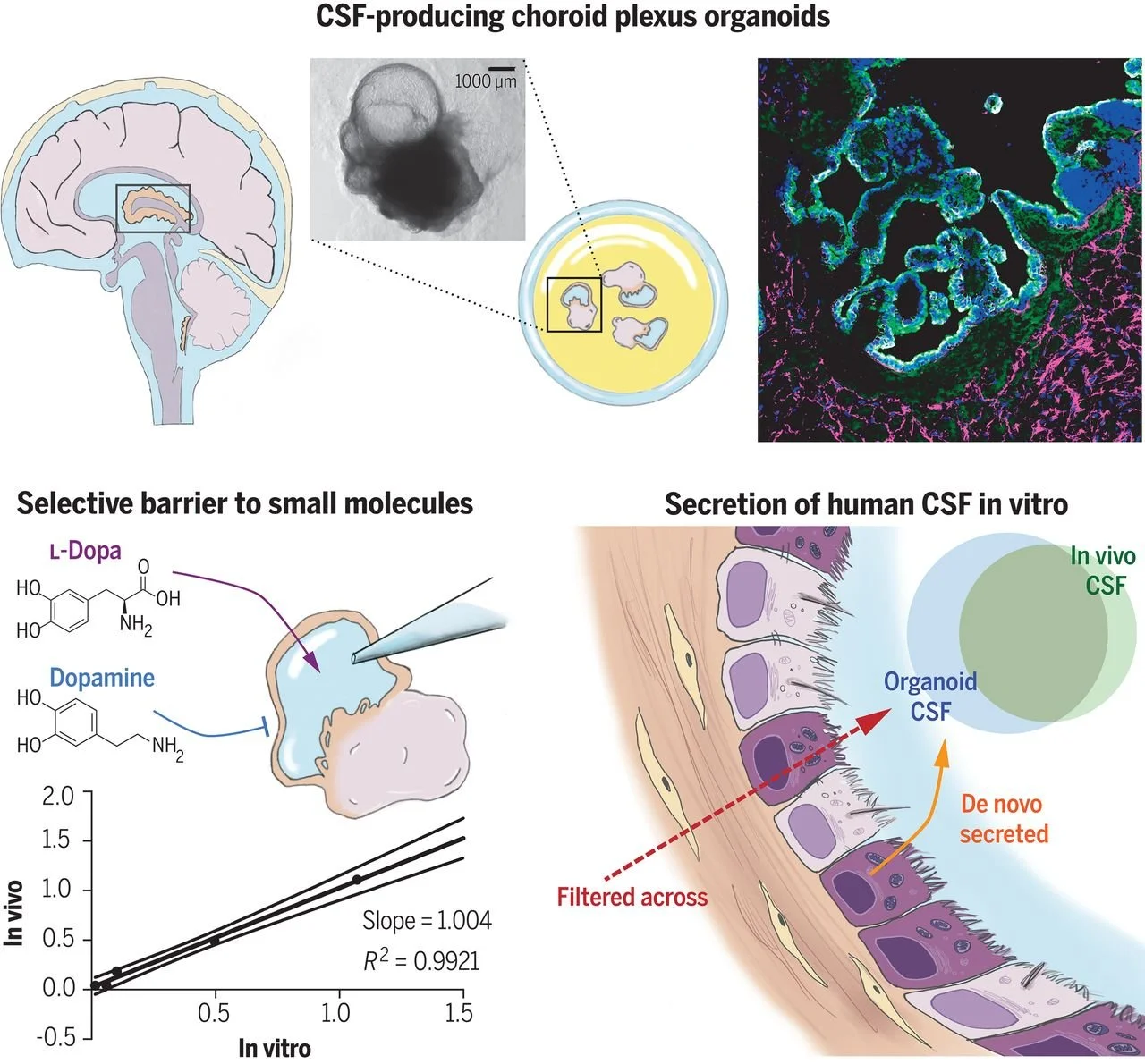
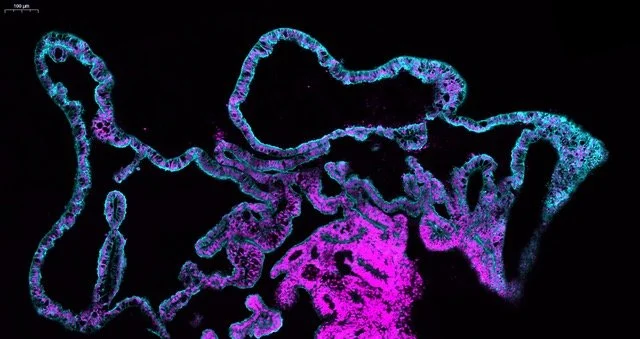
Human CNS barrier-forming organoids with cerebrospinal fluid production
Laura Pellegrini, Claudia Bonfio, Jessica Chadwick, Farida Begum, Mark Skehel, Madeline A. Lancaster
Cerebrospinal fluid (CSF) is a vital liquid, providing nutrients, signaling molecules, and clearing out toxic byproducts from the brain. The CSF is produced by the choroid plexus (ChP), a protective epithelial barrier that also prevents free entry from the blood. Here, we establish human ChP organoids with a selective barrier and CSF-like fluid secretion in self-contained compartments. We show that this in vitro barrier exhibits the same selectivity to small molecules as in vivo, and that ChP-CSF organoids can predict CNS permeability of novel compounds. The transcriptomic and proteomic signature of ChP-CSF organoids reveal a high degree of similarity to in vivo. Finally, the intersection of single cell transcriptomics and proteomic analysis uncovers key human CSF components produced by previously unidentified specialized epithelial subtypes.
Cell Stem Cell | 2020
SARS-CoV-2 Infects the Brain Choroid Plexus and Disrupts the Blood-CSF Barrier in Human Brain Organoids
Laura Pellegrini, Anna Albecka, Donna L. Mallery, Max J. Kellner, David Paul, Andrew P. Carter, Leo C. James,and Madeline A. Lancaster
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) virus, leads to respiratory symptoms that can be fatal. However, neurological symptoms have also been observed in somepatients. The cause of these complications is currently unknown. Here,we use human-pluripotent-stem-cell-derived brain organoids to examine SARS-CoV-2 neurotropism. We find expression of viral receptor ACE2 in mature choroid plexus cells expressing abundant lipoproteins, but not in neurons or other cell types. Wechallenge organoids with SARS-CoV-2 spike pseudovirus and live virus to demonstrate viral tropism for choroid plexus epithelial cells but little to no infection of neurons or glia.We find that infected cells are apolipoprotein-and ACE2-expressing cells of the choroid plexus epithelial barrier. Finally, we show that infection with SARS-CoV-2 damages the choroid plexus epithelium, leading to leakage across this important barrier that normally prevents entry of pathogens, immune cells, and cytokines into cerebrospinal fluid and the brain.
Alzheimer's & Dementia | 2023
Choroid plexus protein turnover in human choroid plexus organoids recapitulates turnover in humans measured using stable isotope labeling kinetics (SILK)
Tatiana A. Giovannucci, Claire A. Leckey, Eleanor Moncur, Kanza Tariq, Lewis Thorne, Laurence Watkins, Ahmed Toma, Nick C Fox, Randall J. Bateman, Kevin Mills, Laura Pellegrini, Ross W. Paterson
The choroid plexus secretes protein-rich cerebrospinal fluid (CSF). CSF turnover is a key mechanism for protein clearance. Neurodegenerative diseases such as AD are characterised by accumulation of excess proteins that form pathological inclusions. CSF flow dysregulation could therefore be involved in the pathophysiology of neurodegenerative diseases (ND) such as Alzheimer’s Disease. A method for measuring CSF turnover in vivo may be achieved by quantitating CSF derived protein kinetics, providing a biomarker tool with to interrogate the relationship between CSF flow disequilibrium and neurodegeneration.
Cell Stem Cell | 2023
Tissue morphology influences the temporal program of human brain organoid development
Ilaria Chiaradia, Ivan Imaz-Rosshandler, Benedikt S. Nilges, Jerome Boulanger, Laura Pellegrini, Richa Das, Nachiket D. Kashikar, and Madeline A. Lancaster
Progression through fate decisions determines cellular composition and tissue architecture, but how that same architecture may impact cell fate is less clear. We took advantage of organoids as a tractable model to interrogate this interaction of form and fate. Screening methodological variations revealed that common protocol adjustments impacted various aspects of morphology, from macrostructure to tissue architecture. We examined the impact of morphological perturbations on cell fate through integrated single nuclear RNA sequencing (snRNA-seq) and spatial transcriptomics. Regardless of the specific protocol, organoids with more complex morphology better mimicked in vivo human fetal brain development. Organoids with perturbed tissue architecture displayed aberrant temporal progression, with cells being intermingled in both space and time. Finally, encapsulation to impart a simplified morphology led to disrupted tissue cytoarchitecture and a similar abnormal maturational timing. These data demonstrate that cells of the developing brain require proper spatial coordinates to undergo correct temporal progression.
Nature | 2020
Androgens increase excitatory neurogenic potential in human brain organoids
Iva Kelava, Ilaria Chiaradia, Laura Pellegrini, Alex T. Kalinka, and Madeline A. Lancaster
The biological basis of male–female brain differences has been difficult to elucidate in humans. The most notable morphological difference is size, with male individuals having on average a larger brain than female individuals, but a mechanistic understanding of how this difference arises remains unknown. Here we use brain organoids to show that although sex chromosomal complement has no observable effect on neurogenesis, sex steroids—namely androgens—lead to increased proliferation of cortical progenitors and an increased neurogenic pool. Transcriptomic analysis and functional studies demonstrate downstream effects on histone deacetylase activity and the mTOR pathway. Finally, we show that androgens specifically increase the neurogenic output of excitatory neuronal progenitors, whereas inhibitory neuronal progenitors are not increased. These findings reveal a role for androgens in regulating the number of excitatory neurons and represent a step towards understanding the origin of sex-related brain differences in humans.
Reviews
Fluids and Barriers of the CNS | 2024
Breakthroughs in choroid plexus and CSF biology from the first European Choroid plexus Scientific Forum (ECSF)
Laura Pellegrini, Violeta Silva‑Vargas, and Annarita Patrizi
The European Choroid plexus Scientific Forum (ECSF), held in Heidelberg, Germany between the 7th and 9th of November 2023, involved 21 speakers from eight countries. ECSF focused on discussing cutting-edge fundamental and medical research related to the development and functions of the choroid plexus and its implications for health, aging, and disease, including choroid plexus tumors. In addition to new findings in this expanding field, innovative approaches, animal models and 3D in vitro models were showcased to encourage further investigation into choroid plexus and cerebrospinal fluid roles.
Current Opinion in Cell Biology | 2021
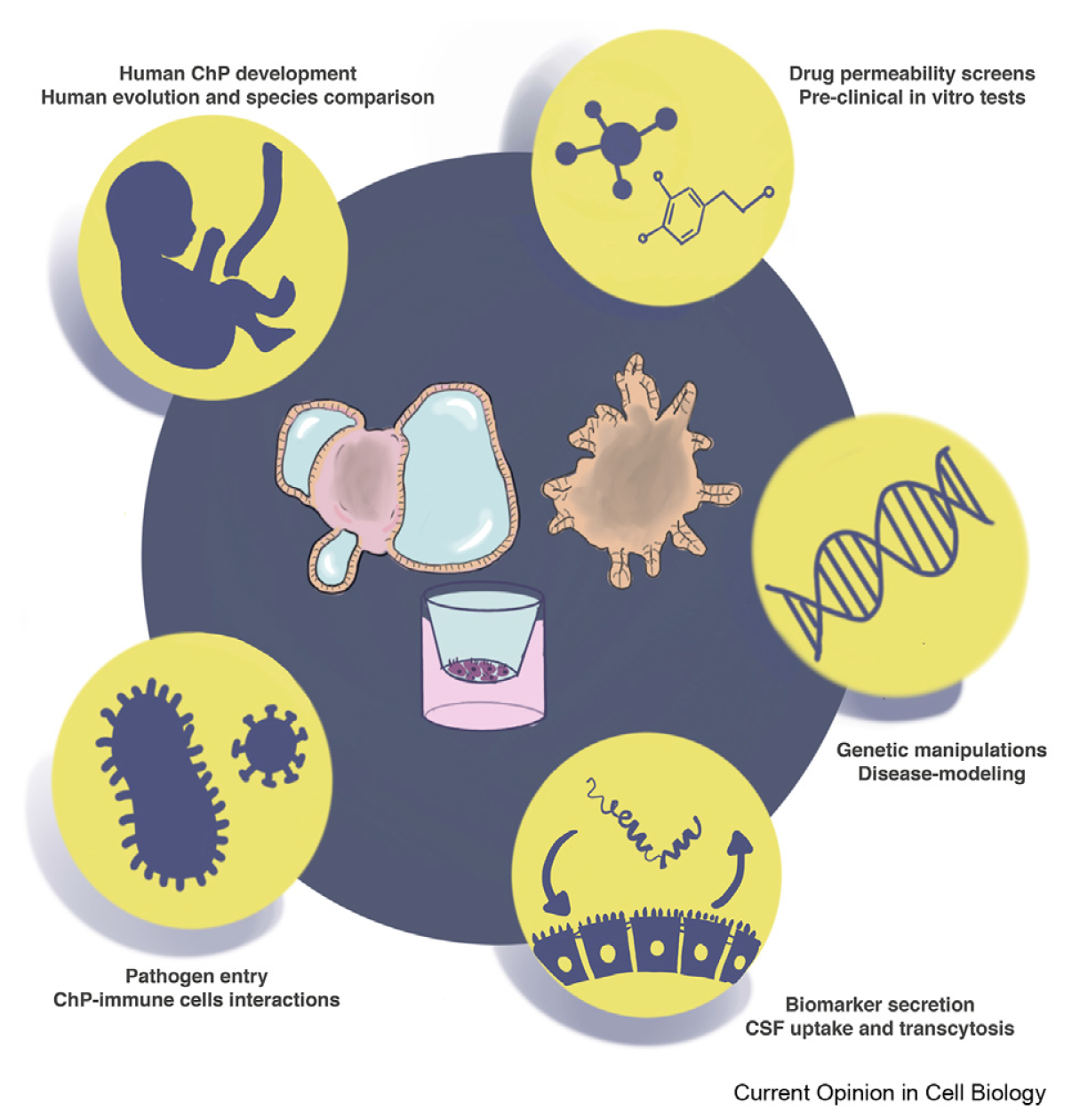
Breaking the barrier: In vitro models to study choroid plexus development
Laura Pellegrini and Madeline A. Lancaster
The choroid plexus is central to normal brain function by secreting cerebrospinal fluid and dynamically regulating its composition throughout development and homoeostasis. Much of our current understanding of this region of the brain comes from studies in animal models. These fundamental studies have shed light on choroid plexus mechanisms of secretion, barrier function and homoeostatic regulation. However, how these specific mechanisms are regulated in the human choroid plexus is much less understood, due to ethical and technical limitations. A number of recent breakthroughs have enabled a new range of techniques and tools for functional characterisation of choroid plexus development and physiology. With the advance of new technologies such as in vivo imaging, singlecell transcriptomics and in vitro three-dimensional cultures we are now able to address a number of outstanding questions in choroid plexus biology. Here, we discuss some of these recent breakthroughs and we focus in particular on how in vitro models can be a powerful tool to study human cerebrospinal fluid secretion and barrier function.
Blog and preview articles
Cell Previews | 2021
Modeling neurodegeneration with mutant-tau organoids
Laura Pellegrini, Madeline A. Lancaster
Greater understanding of the events preceding neurodegeneration is needed to design effective preventive and therapeutic strategies. In this issue of Cell, Bowles et al. (2021) report cerebral organoids that reveal early events in frontotemporal dementia pathogenesis due to mutations in microtubule-associated protein tau (MAPT), shedding light on a novel mechanism involving abnormal splicing and glutamate signaling.
The Node (The company of Biologists) | 2020
From mysterious cysts to CSF-in-a-dish
Laura Pellegrini
The article discusses the serendipitous development of a new method for culturing human brain fluid, also known as cerebrospinal fluid (CSF), in the lab. This fluid is essential for brain function and has been difficult to study in vitro (in a dish) due to the complexities of the brain. This in vitro organoid model discussed here is characterised by formation of cystic compartment containing CSF-like fluid. These cysts have been a bit of a mystery but by studying their composition, scientists hope to develop a more accurate and human-specific system for studying CSF in a lab setting. This new approach could be valuable for researchers investigating neurological diseases and developing new treatments.